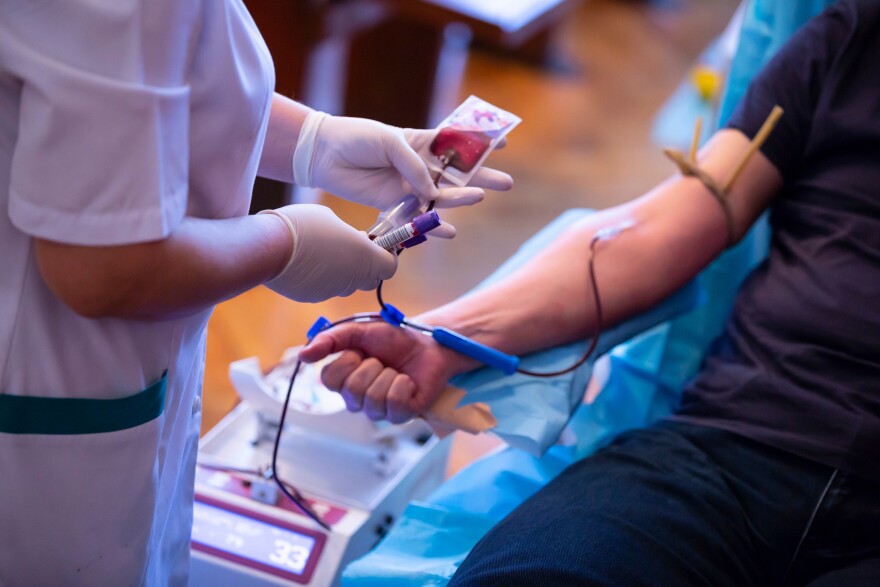
Michigan’s attorney general is part of a multi-state coalition urging the FDA to finally change a blood donation policy that’s long been labeled “discriminatory” against men who have sex with men.
Since the AIDS epidemic of the 1980s, the FDA has issued blanket restrictions on blood donations from men who have sex with men. What was once a lifetime ban became a year-long abstinence requirement, before becoming a three-month abstinence requirement due to a severe blood shortage in 2020.
But earlier this year, the FDA issued new draft guidance. It would ask all donors, regardless of sexual orientation or gender, about whether they’ve engaged in high-risk sexual activity in the past three months.
Now nearly half the states' attorneys general, including Michigan’s, are using the 60-day public comment period to urge the FDA to permanently implement this change.
In a statement last month, the American Red Cross said it supported the change, but that updating the policy would take time.
“The U.S. Food and Drug Administration’s draft guidance which proposes new blood donor-eligibility criteria using a gender-inclusive, individual donor assessment — regardless of sexual orientation — to reduce the risk of transfusion transmitted HIV is a critical step forward toward that goal.
The Red Cross is committed to making these eligibility changes as quickly as possible, however, development of a new industry health history questionnaire and implementation of the guidance requires coordination with multiple organizations including the FDA and the AABB. We will have more information on this timeline in the weeks ahead.”